Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only established curative treatment option for sickle cell disease (SCD). In adult SCD patients, myeloablative conditioning is associated with significant toxicity, primarily because of cumulative organ damage. Recently, matched sibling donor (MSD) transplantation with non-myeloablative conditioning (alemtuzumab/3 Gy total body irradiation (TBI)) showed promising outcomes in adult SCD patients. This chemotherapy-free regimen, typically resulting in mixed chimerism, is associated with mild toxicity with no reports of graft-versus-host disease (GvHD). Despite mixed chimerism, the red blood cell (RBC) phenotype is completely donor-derived. However, in a large series, 13% of patients experienced graft failure. Another 8.2% of patients could not stop their immunosuppression (sirolimus) after one year, because of low donor T-cell chimerism (<50%). Reported mean donor T-cell chimerism at 12-months post-transplantation was 48% (Br J Haematol. 2021 Feb;192(4): 761-768).
We hypothesized, that the addition of azathioprine and hydroxyurea as preconditioning to the alemtuzumab/TBI conditioning regimen will reduce the risk of graft failure and improve donor T-cell chimerism, enabling successful withdrawal of sirolimus in all patients.
Aim: To investigate the effects of adding azathioprine/hydroxyurea preconditioning to alemtuzumab/TBI conditioning on donor chimerism levels and the occurrence of graft failure in adult SCD patients receiving non-myeloablative MSD-HSCT.
Methods: Adult SCD patients with an available HLA-identical sibling donor were eligible for this treatment. All patients received three months of azathioprine 150mg qd and hydroxyurea 25mg/kg qd preconditioning. After exchange transfusion (target HbS <30%), conditioning with alemtuzumab 1mg/kg total dose and 3Gy TBI started on day -7, followed by unmanipulated peripheral hematopoietic stem cell infusion on day 0. Sirolimus as GvHD and rejection prophylaxis started on day -1.
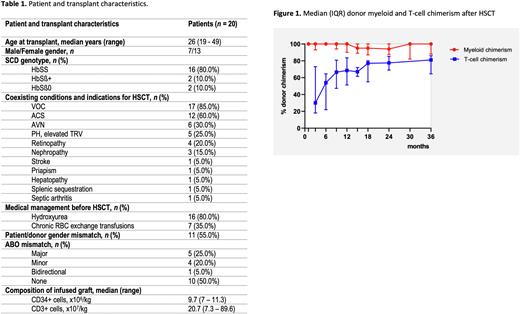
Results: Twenty SCD patients (median age 26 (range 19-49) years) were transplanted with a median follow-up of 24.5 months (range 1-52 months). Patient and transplant characteristics are shown in Table 1. Median donor myeloid and T-cell chimerism of engrafted patients was 100% (range 87-100%) and 68.5% (range 37-89%) at 12-months post-transplantation and 94% (range 86-100%) and 78.5% (range 68-88%) at 18-months post-transplantation, respectively (Figure 1). These donor T-cell chimerism percentages are higher than previously reported with alemtuzumab/TBI conditioning only. All engrafted patients had a corrected SCD phenotype with normalized hemoglobin levels. Importantly, all patients with a follow-up of ≥ 12 months (n=12) could successfully taper and stop sirolimus without decreases in donor chimerism. One year estimated disease-free survival and overall survival were 94.7%. and 93.3%, respectively. One patient (5%) experienced graft failure without autologous regeneration and subsequently died after a series of complications, including EBV reactivation, post-transplantation lymphoproliferative disease, and systemic aspergillosis. Acute gastrointestinal GvHD grade 2 occurred in one patient (5%) and resolved quickly after treatment with prednisolone.
Summary/Conclusion: Azathioprine/hydroxyurea preconditioning prior to alemtuzumab/TBI resulted in improved donor T-cell chimerism, potentially reducing the risk of graft failure after non-myeloablatieve MSD transplantation in SCD patients. Importantly, all engrafted patients reached donor T-cell chimerism >50% and were able to stop immunosuppressives as scheduled.
Disclosures
Biemond:Celgene: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Modus Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanquin: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nur:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal